Introduction
Second-generation FLT3i demonstrated composite CR rates (CRc) of 45-55% in pts with relapse/refractory (R/R) FLT3-mutated AML in phase II/III (ADMIRAL, QUANTUM-R) trials. However, >85% of pts treated in these trials were prior FLT3i naïve as these trials enrolled most of their pts prior to midostaurin approval. The response rates to sequential FLT3i exposure remain poorly defined. The goal of this analysis was to provide benchmark response rates to second and even potentially third sequential FLT3i exposures.
Methods
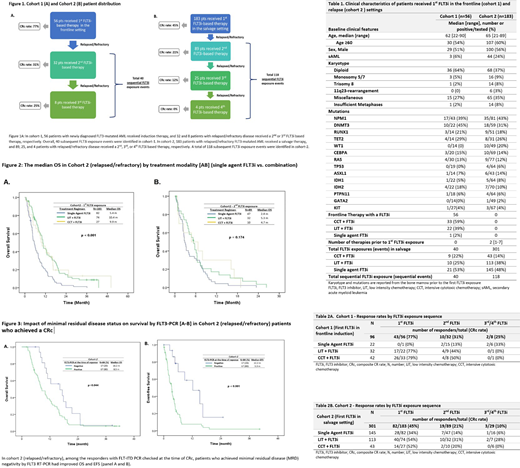
We retrospectively reviewed adult pts with FLT3-mutated AML treated between Jan 2006 and Dec 2019 at our institution. Single agent FLT3i, FLT3i-based combinations with cytotoxic chemotherapy (CCT) and with low intensity therapy (LIT) (hypomethylating agent or low-dose cytarabine based combinations) were included. Cohort 1 (Figure 1A) included pts who received first FLT3i-based therapy in the "frontline induction" followed by post-induction FLT3i-based salvage therapies. Cohort 2 (Figure 1B) included pts who received their first FLT3i-based therapy in "salvage" followed by sequential FLT3i based therapies in subsequent salvages.
Results
A total of 239 pts with FLT3-ITD and/or FLT3-D835 mutated AML who received FLT3i based treatments were identified (Table 1).
Cohort 1 - First FLT3i exposure in frontline setting
In frontline pts who received a FLT3i (cohort 1), the CRc rates with the first "induction" (n=56), and post-induction salvage: second (n= 32) and third FLT3i-based (n= 8) therapies, were 77%, 31%, and 25% respectively (Table 2A). The median overall survival (OS) with the first, second and third FLT3i-based therapies were 16.7 months, 6.0 months, and 1.4 months, respectively.
Cohort 2 - First FLT3i exposure in salvage setting
In pts receiving a FLT3i-based therapy for the first time in a R/R AML setting (i.e. no FLT3i with induction) (cohort 2), the CRc rates and median OS were 45%, 21%, and 10%, and 7.9 months, 4.0 months, and 4.1 months with the first (n=183), second (n=89), and third/fourth (n=29) sequential FLT3i-based therapies, respectively (Table 2B).
Single-agent versus Combination FLT3i-based therapies
In cohort 1, in the post-induction salvage setting, the CRc rates with single-agent FLT3i (n=21) versus combinations (n=19) were 19% versus 42%, respectively. (Table 2A). In cohort 2, the CRc rates with single-agent FLT3i (n=82) versus FLT3i-based combinations (n=101) in first FLT3i exposure were 34% versus 53%, respectively, and with single agent FLT3i (n=63) versus FLT3i-based combinations (n=55) in the second/third/fourth sequential FLT3i exposures were 13% versus 25%, respectively (Table 2B). The median OS with the first FLT3i-based therapy in salvage AML was 5.4, 10.4, and 9.9 months with single-agent, LIT, and CCT FLT3i-based therapies, respectively (P<0.001) (Figure 2A). Median OS with the second FLT3i-based therapy exposure in salvage AML was 2.8, 5.3, and 4.7 months, with single-agent, LIT, and CCT FLT3i-based therapies, respectively (P=0.174) (Figure 2B).
Impact of Minimal Residual Disease at CRc in Cohort 2
In the salvage AML (cohort 2), 104 of 301 achieved CRc, and 84 of 104 (80%) of these pts had serial FLT3-ITD/TKD PCR checked on the bone marrow at baseline and at CRc. Pts who achieved MRD negativity by FLT3-PCR had improved OS (16.3 versus 8.5 months, P=0.04) and event free survival (EFS) (12.2 versus 3.3 months, P<0.001) (Figure 3A-B). Achievement of MRD-negativity by MFC at CRc was however not associated with a significant impact on OS (9.8 vs 10.7 months, P=0.55) nor EFS (4 vs 3.4 months, P=0.19).
Conclusion
The CRc rates and median OS dropped with sequential FLT3i exposure, from induction to post-induction salvage (cohort 1) and sequentially in subsequent salvages (cohort 2). FLT3i combinations demonstrated improved CRc rates and improved OS compared with single agent FLT3i's in all similar FLT3i exposure settings. Achievement of MRD negativity by FLT3-PCR improved OS in R/R setting. These data provide benchmark expectations in the "post-midostaurin" and now "post-gilteritinib" era for clinical trials evaluating combinations of FLT3i's with chemotherapy, hypomethylating agents, venetoclax, and triplets of hypomethylating agents with venetoclax and FLT3i's in R/R FLT3-AML, a majority of whom will have received one or more prior FLT3 TKI therapies.
Yilmaz:Pint Pharma: Honoraria; Pfizer: Research Funding; Daicho Sankyo: Research Funding. Kadia:Pfizer: Honoraria, Research Funding; Novartis: Honoraria; BMS: Honoraria, Research Funding; Celgene: Research Funding; Incyte: Research Funding; Genentech: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Cyclacel: Research Funding; Astellas: Research Funding; JAZZ: Honoraria, Research Funding; Astra Zeneca: Research Funding; Ascentage: Research Funding; Pulmotec: Research Funding; Cellenkos: Research Funding; Amgen: Research Funding. DiNardo:Agios: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria; Jazz: Honoraria; Novartis: Consultancy; Notable Labs: Membership on an entity's Board of Directors or advisory committees; MedImmune: Honoraria; Syros: Honoraria; Takeda: Honoraria. Borthakur:Incyte: Research Funding; PTC Therapeutics: Research Funding; Nkarta Therapeutics: Consultancy; BioTherix: Consultancy; Treadwell Therapeutics: Consultancy; Argenx: Consultancy; FTC Therapeutics: Consultancy; Curio Science LLC: Consultancy; Oncoceutics: Research Funding; Xbiotech USA: Research Funding; Polaris: Research Funding; AstraZeneca: Research Funding; BMS: Research Funding; BioLine Rx: Research Funding; GSK: Research Funding; Jannsen: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; BioLine Rx: Consultancy; PTC Therapeutics: Consultancy; Cyclacel: Research Funding. Konopleva:Eli Lilly: Research Funding; Calithera: Research Funding; Stemline Therapeutics: Consultancy, Research Funding; Sanofi: Research Funding; Forty-Seven: Consultancy, Research Funding; Agios: Research Funding; AstraZeneca: Research Funding; Kisoji: Consultancy; Genentech: Consultancy, Research Funding; Amgen: Consultancy; F. Hoffmann La-Roche: Consultancy, Research Funding; Ascentage: Research Funding; AbbVie: Consultancy, Research Funding; Rafael Pharmaceutical: Research Funding; Cellectis: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Ablynx: Research Funding. Jabbour:Amgen: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding. Garcia-Manero:Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Consultancy; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Onconova: Research Funding; Merck: Research Funding; Novartis: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; Amphivena Therapeutics: Research Funding; H3 Biomedicine: Research Funding; AbbVie: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Pemmaraju:Plexxikon: Research Funding; Cellectis: Research Funding; AbbVie: Honoraria, Research Funding; Pacylex Pharmaceuticals: Consultancy; SagerStrong Foundation: Other: Grant Support; Celgene: Honoraria; Novartis: Honoraria, Research Funding; Samus Therapeutics: Research Funding; Stemline Therapeutics: Honoraria, Research Funding; Incyte Corporation: Honoraria; DAVA Oncology: Honoraria; Roche Diagnostics: Honoraria; Daiichi Sankyo: Research Funding; Affymetrix: Other: Grant Support, Research Funding; MustangBio: Honoraria; LFB Biotechnologies: Honoraria; Blueprint Medicines: Honoraria. Issa:Novartis: Membership on an entity's Board of Directors or advisory committees; Syndax: Research Funding; Celegene: Research Funding. Short:Astellas: Research Funding; Amgen: Honoraria; AstraZeneca: Consultancy; Takeda Oncology: Consultancy, Honoraria, Research Funding. Andreeff:Daiichi-Sankyo; Breast Cancer Research Foundation; CPRIT; NIH/NCI; Amgen; AstraZeneca: Research Funding; Centre for Drug Research & Development; Cancer UK; NCI-CTEP; German Research Council; Leukemia Lymphoma Foundation (LLS); NCI-RDCRN (Rare Disease Clin Network); CLL Founcdation; BioLineRx; SentiBio; Aptose Biosciences, Inc: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Daiichi-Sankyo; Jazz Pharmaceuticals; Celgene; Amgen; AstraZeneca; 6 Dimensions Capital: Consultancy. Cortes:Sun Pharma: Research Funding; BioPath Holdings: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Telios: Research Funding; Astellas: Research Funding; Amphivena Therapeutics: Research Funding; Arog: Research Funding; BiolineRx: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Immunogen: Research Funding; Merus: Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Takeda: Consultancy, Research Funding. Kantarjian:Ascentage: Research Funding; BMS: Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Immunogen: Research Funding; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sanofi: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive biotechnologies: Honoraria; Aptitute Health: Honoraria; BioAscend: Honoraria; Delta Fly: Honoraria; Janssen: Honoraria; Oxford Biomedical: Honoraria; Amgen: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Ravandi:AstraZeneca: Consultancy, Honoraria; Xencor: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Orsenix: Consultancy, Honoraria, Research Funding. Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal